I recently came across a Cobalt-57 (Co-57) source sent as Co-60. That’s a lucky wrong labeling case that gived me this source for free.In this post, I’ll explore how Co-57 is produced and its important industrial roles.

What is Cobalt-57?
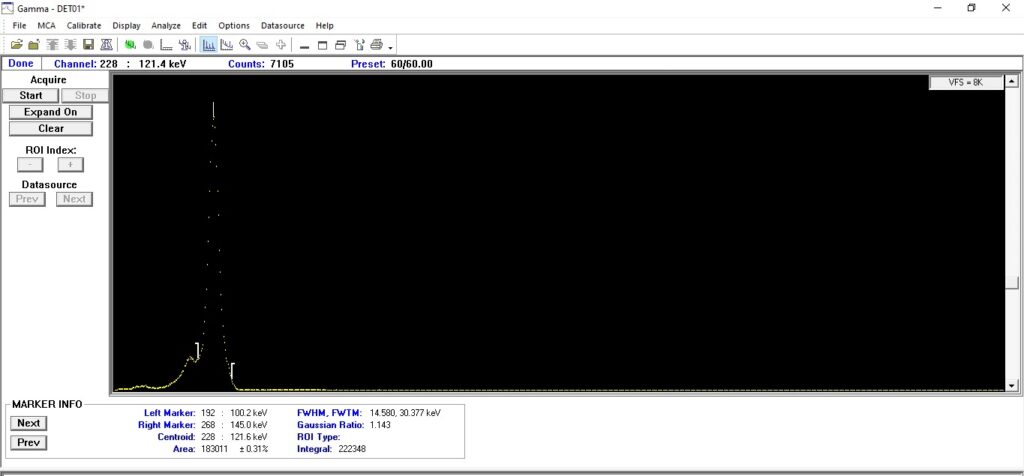
Cobalt-57 is a radioactive isotope of cobalt with a half-life of approximately 271.8 days. It decays via electron capture to stable iron-57 (Fe-57), emitting gamma radiation in the process. The primary gamma photons produced have energies of 122 keV and 136 keV, making Co-57 an excellent source for various specialized applications.
How is Cobalt-57 Produced?
The production of Co-57 involves the irradiation of natural cobalt (Co-59) with protons in a cyclotron or through neutron capture in a reactor. This process converts Co-59 into Co-57 through the following reactions:
- Cyclotron production: Co-59+p→Co-57
- Reactor production: Co-59+n→Co-60→Co-57+2n
After irradiation, the Co-57 is separated and purified for use.
Industrial Applications of Cobalt-57
Co-57 has several significant industrial applications, albeit different from its more famous cousin, Co-60:
- Medical Diagnostics: Co-57 is extensively used in nuclear medicine, particularly in diagnostic imaging. It is a key component in radiopharmaceuticals, like vitamin B12 labeled with Co-57, used in Schilling tests to diagnose vitamin B12 absorption issues.
- Calibration of Gamma Cameras: Due to its specific gamma emissions, Co-57 is ideal for calibrating gamma cameras used in medical imaging. This ensures accurate imaging results and reliable diagnostics.
- Scientific Research: Co-57 is used in Mössbauer spectroscopy, a technique that studies the resonant absorption of gamma rays and provides detailed information about the atomic and magnetic structure of materials.